Cardiovascular Research LLP
Start-up for Research and Development of Medical Devices for Endovascular Surgery and Endoprosthesis Replacement.
Cardiovascular Research LLP was incorporated in 2022 dedicated to helping interventional cardiologists and interventional radiologists perform successful coronary stenting procedures.
Cardiovascular Research LLP
The specialist for medical implants in the CIS countries

Cardiovascular Research LLP
The specialist for medical implants in the CIS countries Our focus lies in the field of stents up to endoprostheses.
We have more than 20 years of experience in the manufacturing, distribution, and servicing of medical implants
By 2026, we aim to become the leading manufacturer of endoprostheses in the CIS region.
2021
Initiation of strategic planning and preparation for the company's establishment.
2022
Company establishment.
2022
Commencement of stent and endoprosthesis distribution in Kazakhstan.
2022
Planning for the in-house manufacturing of endoprostheses (knee and hip implants).
Manufacturing for Endoprostheses – Current Status
• 2022 Completion of prostheses development.
• 2022 Preparation of specifications for the equipment of the manufacturing facility.
• 2022 Finalization of the location for manufacturing in Kazakhstan.
• 2023 Nomination of suppliers for the equipment.
• 2023 Commencement of the procurement of surgical instruments for knee and hip joint implantation.
• 2022 Completion of prostheses development.

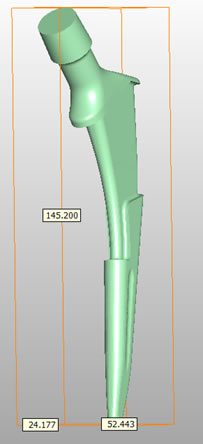
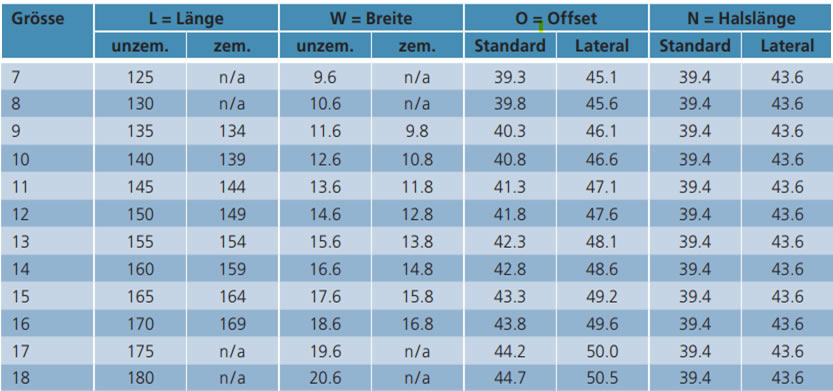
• Cemented and cementless Technical data
• Material: CoCrMo (ISO 5832-4)
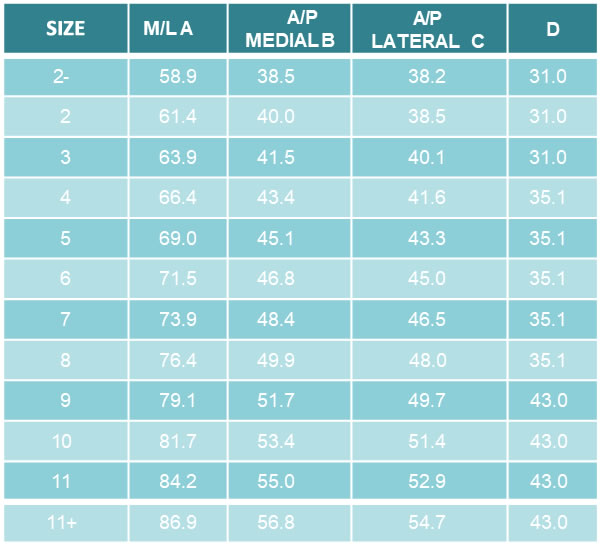
• Sizes: Standard: 7.5 – 20
• Lateral: 7,5 – 15
• CCD angle:135
 Volume [mm³]: 19188.5
Surface area [mm²]: 7284.4
Volume [mm³]: 19188.5
Surface area [mm²]: 7284.4
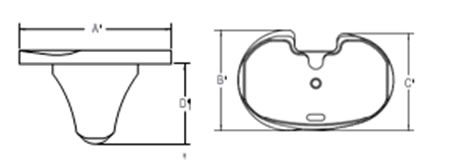
Technical data
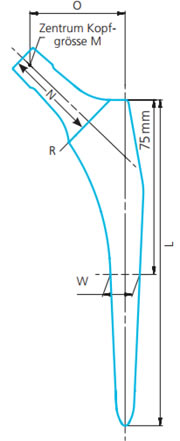 O: Offset
O: OffsetW: Width
L: Length
R: Resection line
N: Neck length
• The weight of a single prosthesis component is approximately 250-400 grams made of CoCr alloy
• A "garland" of about 20 parts is formed for casting.
• This results in a total casting weight of approximately 10-12 kg with the sprues.
• Casting mold material: ceramic mold
Technical femoral head

• Ø 28, 32 und 36 mm
• Taper 12/14
• Neck lengths: 3, +0, +4, +8, +12, +16 mm
• Material CoCrMo
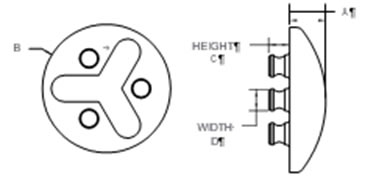
Technical tray
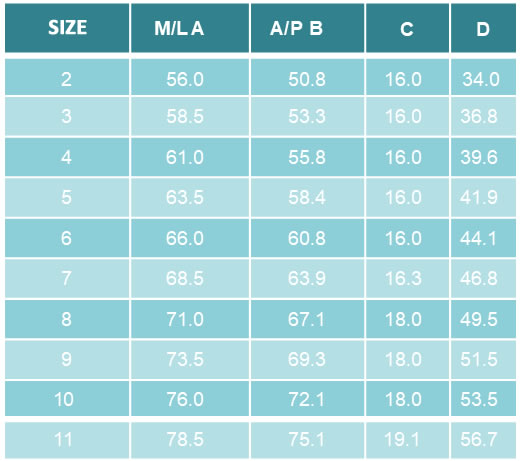
• Material CoCrMo
• Coating
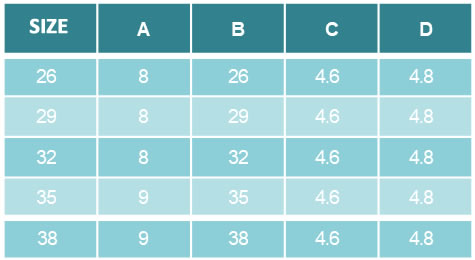
• Sizes: 40 – 68 mm
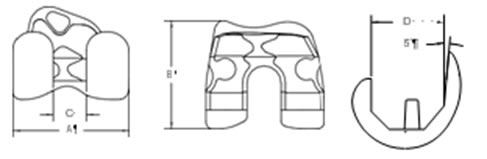
• zementierte und zementfrei
• Material: CoCrMo (ISO 5832-4)
Next steps
• 2023/24 Delivery of equipment.
• 2024 Initiation of prototype manufacturing.
• 2024/25 Completion of certification and approval for production in the CIS market.
• 2025/26 Commencement of distribution in the CIS countries.
• 2026 Increase in production capacity.
• 2027 Expansion of distribution activities to additional countries.

Kazakhstan, Bukhar-Zhyrau Blvd 26/1, Almaty 050021, Business Center "Evolution"
tel, w/p: +7 707 350 3677
email: info@cvr.kz